European Business Summits
info@ebsummit.eu – 0032 (0) 2 645 34 80
TRANSPARENCY REGISTER 268958411031-65
PRIVACY-POLICY | GENERAL TERMS OF SALES

“Now that the pandemic is more or less under control, it is the time for addressing major public health issues and especially rare disease research.”
“Rare disease is a very challenging area. We must collaborate with all sectors at both EU and global level. The EU has already invested quite heavily & will continue to fund research.”
“We are preparing our patients through training to give input that is timely and effective. Meaningful patient input and engagement at all levels are important for clinical trials.”
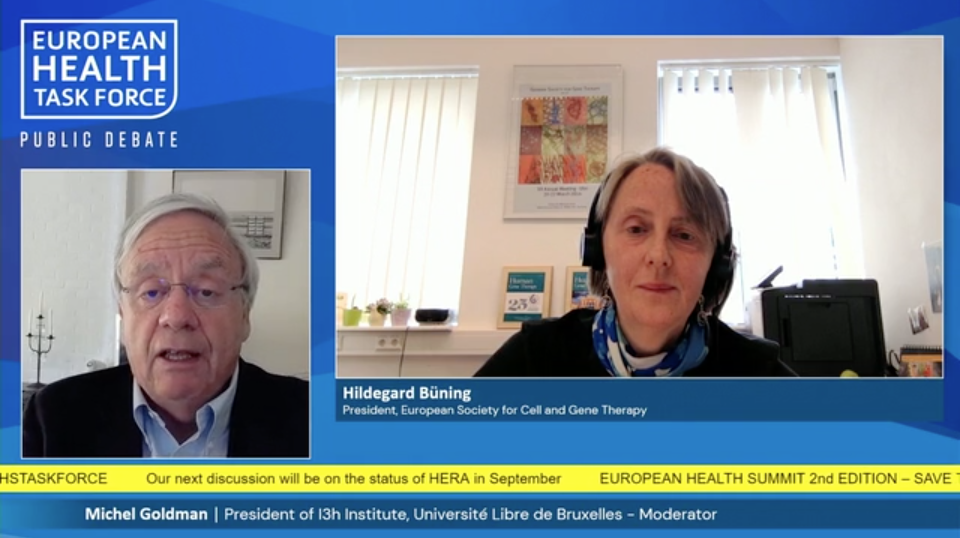
“The Covid-19 pandemic created a sense of urgency which led to an unprecedent level of collaboration, openness & willingness to implement innovative thinking & transformative procedures. Hopefully we will take these lessons learned and apply them to gene therapies.”
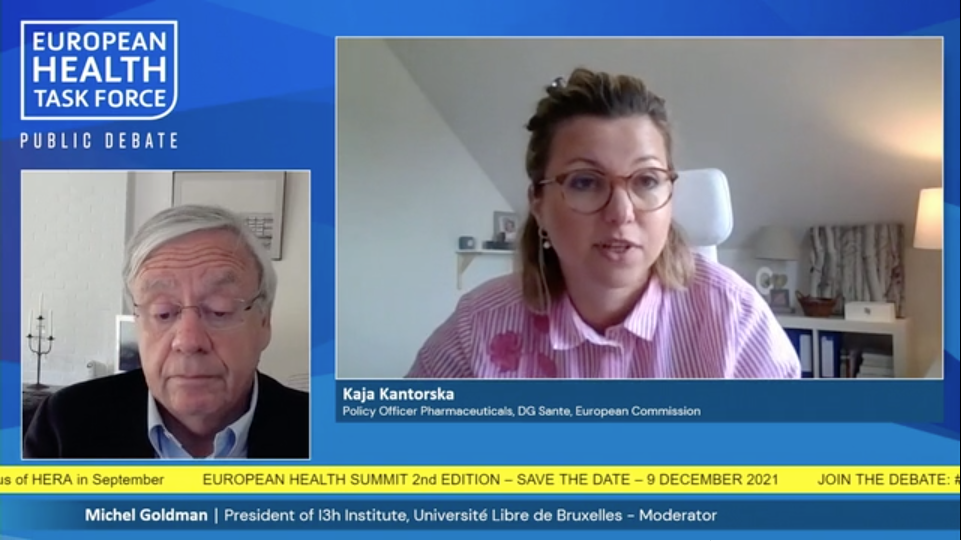
“We are reforming the big pharmaceutical regulation according to three main goals: ensuring timely approuval, enhancing and boosting the development of innovative products and providing better medicine access to patients.”
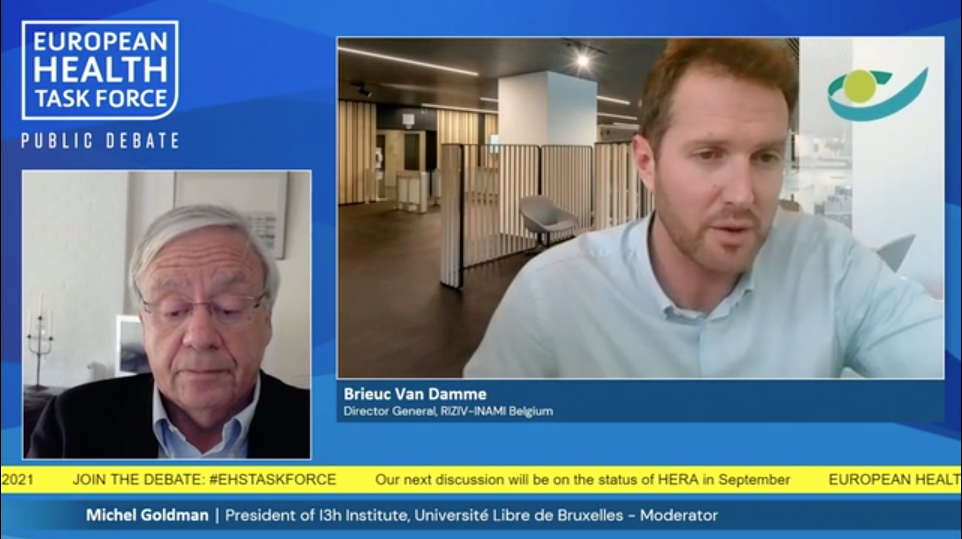
“It is important to facilitate early dialogue between industry, payers & regulators where all actors share their expectations about what they are willing to pay. Contractual pacts can be used to that end.”
“We have to address three issues: access of patients to the therapies, improving the current therapies and dealing with manufacturing issues.”
European Business Summits
info@ebsummit.eu – 0032 (0) 2 645 34 80
TRANSPARENCY REGISTER 268958411031-65
PRIVACY-POLICY | GENERAL TERMS OF SALES

This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.